UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (date of earliest event reported) June 7, 2019
electroCore, Inc.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
001-38538 (Commission File Number) |
20-3454976 (I.R.S. Employer Identification Number) |
150 Allen Road, Suite 201
Basking Ridge, NJ 07920
(Address of principal executive offices and zip code)
(973) 290-0097
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Title of each class |
Trading symbol(s) |
Name of each exchange on which registered | ||
| Common Stock, Par Value $0.001 Per Share | ECOR | NASDAQ Global Select Stock Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item 8.01 Other Events
On June 7, 2019, members of management of electroCore, Inc. (the “Company”) intend to make a presentation at the Company’s annual meeting of shareholders. A copy of the presentation is filed herewith as Exhibit 99.1 and incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit No. |
Description of Exhibit | |
| 99.1 | Annual Meeting Presentation | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| electroCore, Inc. | ||||
| June 7, 2019 | /s/ Brian Posner | |||
| Brian Posner | ||||
| Chief Financial Officer | ||||

Annual Meeting of Shareholders 06/07/2019 Exhibit 99.1

Disclaimers This presentation contains "forward-looking" statements. These statements identify substantial risks and uncertainties and relate to future events such as execution of our strategy or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “could,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” or “continue,” and similar expressions, whether in the negative or affirmative. These statements are only predictions, may be inaccurate, and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially. In evaluating these statements, you should specifically consider various factors, including the risks outlined in our filings with the Securities and Exchange Commission (SEC), including, without limitation, our periodic and current SEC reports. These factors may cause our actual results to differ materially from any forward-looking statement. Although we believe that the expectations reflected in the forward-looking statements are reasonable, our future results, operational activities, levels of activity, performance or achievements may differ from our expectations. Other than as required by law, we do not undertake to update any of the forward-looking statements after the date of this presentation, even though our situation may change in the future. Important Additional Information and Where to Find It The Company, its directors, director nominees and certain of its executive officers are participants in the solicitation of proxies from the Company’s shareholders in connection with the Company’s 2019 Annual Meeting. The Company has filed a definitive proxy statement and proxy card with the SEC in connection with its solicitation of proxies from the Company’s shareholders. SHAREHOLDERS OF THE COMPANY ARE STRONGLY ENCOURAGED TO READ SUCH PROXY STATEMENT, ACCOMPANYING PROXY CARD AND ALL OTHER DOCUMENTS FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY AS THEY CONTAIN IMPORTANT INFORMATION. Information regarding the identities of the Company’s directors, director nominees and executive officers, and their direct or indirect interests, by security holdings or otherwise as of the dates set forth in the proxy statement, are set forth in the proxy statement and other materials filed with the SEC in connection with the 2019 Annual Meeting. Shareholders can obtain the proxy statement, any amendments or supplements to the proxy statement, and any other documents filed by the Company with the SEC at no charge at the SEC’s website at www.sec.gov. These documents are also available at no charge at the Company’s website at www.electrocore.com in the “Investor Relations” section under “SEC Filings.”

Meeting agenda 1. State of the company, GROWTH MODE! Extended cash runway Current revenue growth opportunities Accelerate revenue growth through payer agreements Clinical program focused on label expansion and commercial growth 2019-2020 Goals and Objectives 2. 3. 4. 5. 6.

Patient testimonials Patient: "Thanks for saving my Thanksgiving Day". I woke up with the beginnings of a migraine and was worried that my day with family and company would be ruined if I had a migraine all day. Then I remembered that I had Gammacore. I was skeptical that it would work but I did exactly what was discussed in my training. Within 20 minutes I was pain-free! I am absolutely ecstatic over Gammacore.” Patient: “I am so, so grateful for the knowledge, time and research that went into the GammaCore Sapphire. It is a Godsend to those of us who suffer from migraines. I am 62 years old and I have had migraines off and on my entire life.” Patient: “I love your product GammaCore and am grateful every day for it. Your investors, your employees, everyone involved needs to know GammaCore is the real thing. It gives people their lives back. Thank you." Patient: "My husband and I believe in this product. We are adamant about the future of this product. We have invested in this product. We appreciate how much you have accepted our feedback. My doctor is a firm believer in this product as well! We also desperately want our daughter to access Gamma Core because we think it would make a difference for her more so than even myself.”

State of the company, GROWTH MODE! We are starting to see revenue growth across several of the channels available to us Optimized sales force size to focus on near term revenue opportunities ~20% of targeted Headache specialists are routinely prescribing gammaCore There is a core group of early adopters prescribing gammaCore We have increased our focus on regional payers to accelerate reimbursement and sales growth Regional plans that manage 106m lives We have streamlined the organizational structure and clinical development plan to reduce cash burn Anticipated cash runway into 2021

Extended cash runway

Extended cash runway – recent actions Reduced annual operating costs by ~$20 million, will continue to look for efficiencies Reduced headcount from 91 to 55 Reduced the number and scope of clinical projects Extended cash runway to 2021 Increased focus on near-term revenue opportunities with the least cost as we contract for greater insurance coverage
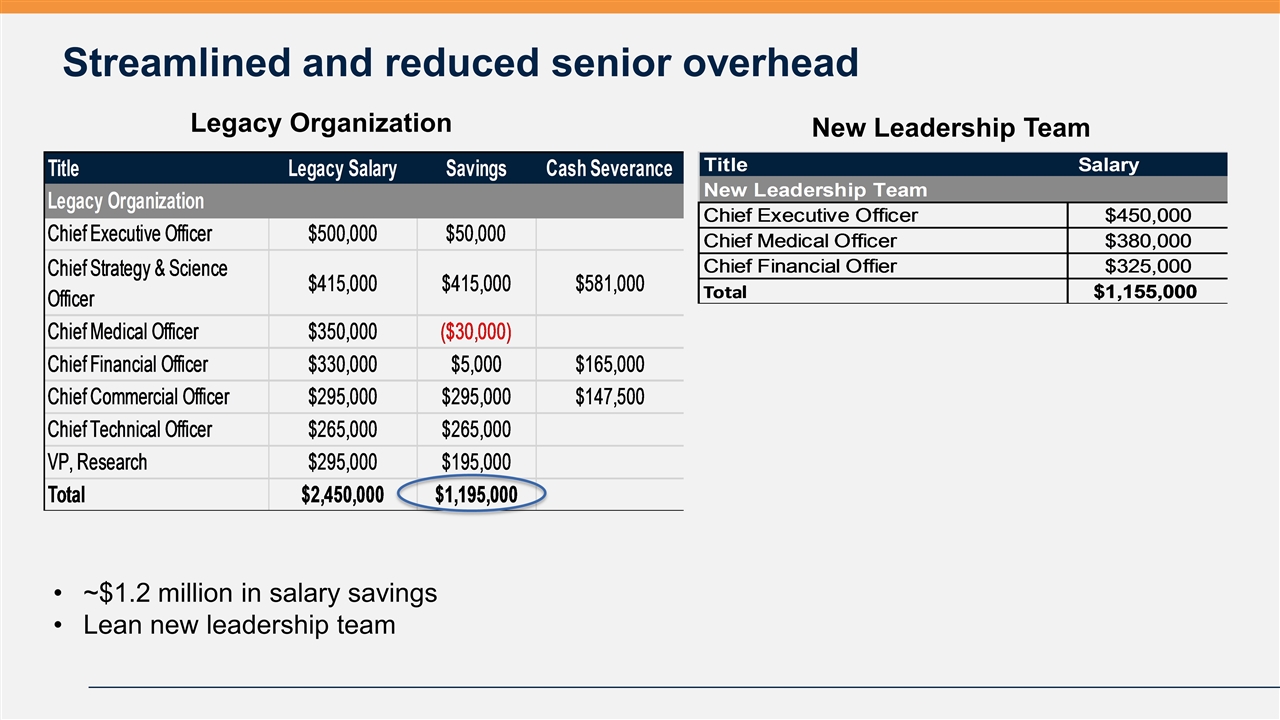
Streamlined and reduced senior overhead New Leadership Team Legacy Organization ~$1.2 million in salary savings Lean new leadership team Title Legacy Salary Savings Cash Severance Legacy Organization Chief Executive Officer $,500,000 $50,000 Chief Strategy & Science Officer $,415,000 $,415,000 $,581,000 Chief Medical Officer $,350,000 $,-30,000 Chief Financial Officer $,330,000 $5,000 $,165,000 Chief Commercial Officer $,295,000 $,295,000 $,147,500 Chief Technical Officer $,265,000 $,265,000 VP, Research $,295,000 $,195,000 Total $2,450,000 $1,195,000 Title Salary New Leadership Team Chief Executive Officer $,450,000 Chief Medical Officer $,380,000 Chief Financial Offier $,325,000 Total $1,155,000
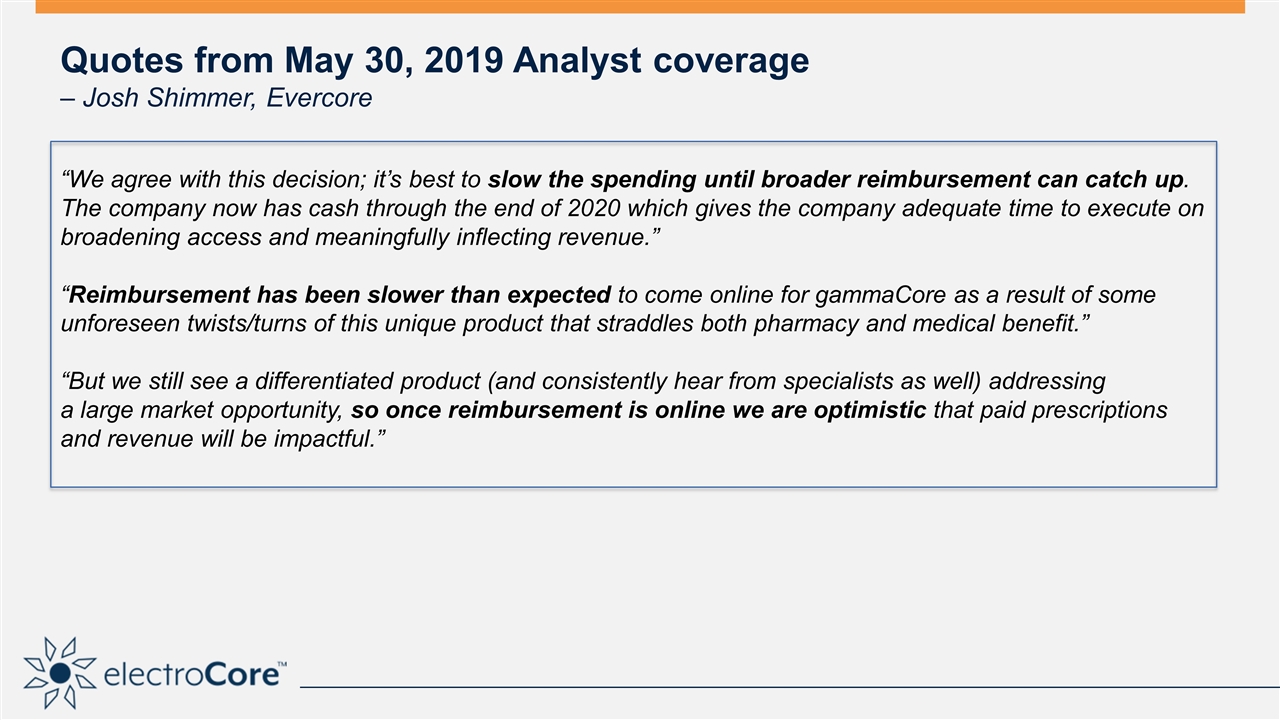
Quotes from May 30, 2019 Analyst coverage – Josh Shimmer, Evercore “We agree with this decision; it’s best to slow the spending until broader reimbursement can catch up. The company now has cash through the end of 2020 which gives the company adequate time to execute on broadening access and meaningfully inflecting revenue.” “Reimbursement has been slower than expected to come online for gammaCore as a result of some unforeseen twists/turns of this unique product that straddles both pharmacy and medical benefit.” “But we still see a differentiated product (and consistently hear from specialists as well) addressing a large market opportunity, so once reimbursement is online we are optimistic that paid prescriptions and revenue will be impactful.”

Current revenue growth opportunities

Channels with potential for growing revenue now … Driving FSS/VA-DoD contract sales - started on January 15th Enhancing CVS/Aetna agreement Pursuing medical benefit contracting with Highmark BCBS Pulling through the UK – Innovation Technology Programme Award for Cluster Headache Finalizing a Workers Compensation Pain Management Initiative Exploring physician dispensing, direct selling model Exploring reimbursement codes through CMS - opioid legislation includes non-invasive neuromodulation as suggested therapy for reimbursement

VA/DoD ~2 million patients suffering with primary headache Large market opportunity for gammaCore Source: 2018 Data derived from WSI FOIA request re: VA/DoD Total Accessible Market (TAM) Federal Government Market Beneficiary Population Enrolled Sex Migraine Prevalence Total (Est) Migraine Patients Cluster Headache Prevalence Total (Est Cluster Headache Patients Combined Total Lives Veterans Health Administration 8,900,000 Male 8,010,000 6% 480,600 0.40% 32,040 512,640 Female 890,000 18% 160,200 0.08% 712 160,912 Military Health System 9,400,000 Male 4,600,000 6% 276,000 0.40% 18,400 294,400 Female 4,800,000 18% 864,000 0.08% 3,840 867,840 Total Patient Lives 18,300,000 1,780,800 54,992 1,835,792 >95% of these patients are migraineurs

QTD unit sales 3x over Q1 (25 Veterans Hospitals), 29 units in the 1st 3 days of June 2019 - Units sold to VA/MTF by month In June, Cardinal Health agreement was initiated to facilitate bulk ordering for 11 Military Treatment Facilities (active military) Reimbursement started Q2 to-date 173 Units

Filled Rx counts by quarter (55 days elapsed) Filled Prescriptions in Qtr: Prescriptions Filled during that Qtr (New, Renewal, Refill) Elapsed Days: Number of days elapsed from 2019-04-01 until 2019-05-25 Prescriptions in Elapsed Days in Qtr: Number of Prescriptions Filled in the first 55 days of that Qtr Data through 5/25/2019

Accelerate Neurology revenue through national and regional payer partnerships

Prime Therapeutics – Targeting 30m regional lives Prime Therapeutics (PBM) is privately owned and represents the following Blues Plans: Prime’s PBM business model is unique since the plans own the PBM Blue Plan Owners Blue Cross Blue Shield of Alabama Blue Cross Blue Shield of Kansas Blue Cross Blue Shield of Minnesota Blue Cross Blue Shield of Nebraska Blue Cross Blue Shield of North Carolina Blue Cross Blue Shield of North Dakota Blue Cross & Blue Shield of Rhode Island Blue Cross and Blue Shield of Wyoming Florida Blue (BCBS of Florida) Health Care Services Corp. (BCBS of Illinois, Montana, New Mexico, Oklahoma, and Texas Regence Health Plans (Blue Shield Idaho and Washington, BCBS of Oregon and Utah Presented pharmacy & medical term sheets, met with Sr. Director, Pharmacy Full clinical & pharmacoeconomic presentation Contract negotiations

Consumer targeted activation campaign toward highest prescribers Find a Physician Searches in 6 Targeted MSAs Launched Paid Social Media and Paid Search Added Web Banner Advertising (Programmatic)
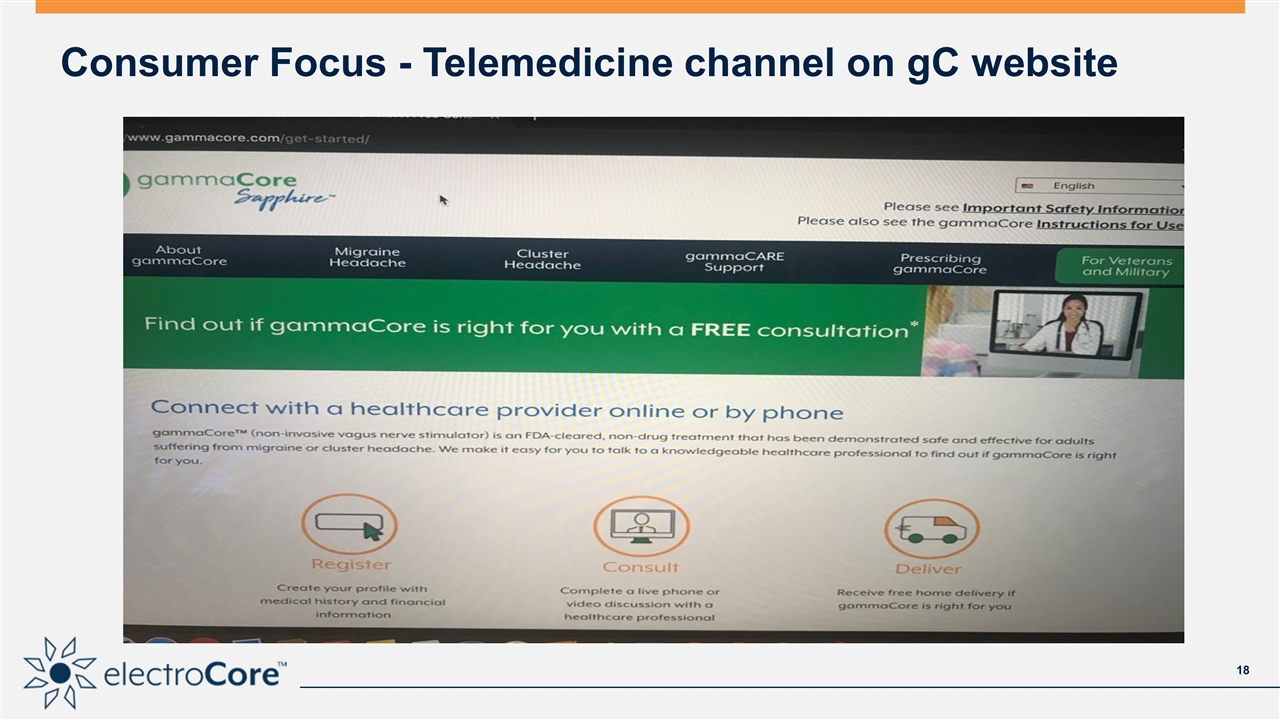
Consumer Focus - Telemedicine channel on gC website

Contracting to “unlock” access to additional growth potential NO PBM denials for coverage to date National PBMs - Express Scripts (Cigna) and CVS Health (Aetna) Express Scripts (ESI) currently manages 83 million members, recently merged with Cigna ESI working with FDB on a Digital Therapeutics module for therapies like gammaCore CVS Health has more than 92 million plan members, recently merged with Aetna electroCore submitted a significantly enhanced bid to CVS

Clinical program focused on label expansion and enhancing commercial growth

Streamlined clinical plan to enhance label expansion and sales GAP-PTH Post-traumatic Headache, under IRB and database review; key to VA focus TR-Venus Treatment of Acute Stroke, enrolling (2/120); majority of funding external grant GENIUS-RA Treatment-Resistant Rheumatoid Arthritis, enrolling (2/40) Premium 2 Migraine Prevention (pivotal), enrolling (129/400) and 74 Randomized ATOM Adolescent Migraine (pivotal), plan to delay until revenue ramp Pivotal Trials Expand indications and use in Headache Low-Cost Pilot Studies Label expansion beyond primary headache

2019 – 2020 Goals and Objectives

Corporate goals and objectives 2H 2019 1H 2020 Drive revenue growth Seeking to secure ESI and Prime Therapeutics, and to enhance CVS agreement Enhance gammaCore’s value proposition Potential US migraine prevention indication Seeking to attain NICE guidance on CH Deliver additional clinical data for payer uptake and increased investment into clinical development Potential to complete: Premium 2, data readout GENIUS-RA, data readout GAP-PTH and VENUS, data readout

Summary Revenue is growing across several currently available channels Regional payer discussions present opportunities for additional revenue growth A streamlined organizational structure and clinical development plan reduces cash burn and extends our runway into 2021 Seeking label expansion and additional clinical data generation to enhance revenue opportunities
