Data Highlighting Non-invasive Vagus Nerve Stimulation (nVNS) for Treatment of Symptoms of Gastroparesis Presented at 2023 American College of Gastroenterology Annual Meeting
Many gastrointestinal disorders can cause nausea and vomiting, of which the most well-known is gastroparesis, a digestive disorder in which the stomach empties slowly.1 The symptoms of gastroparesis can range from mild to severe, requiring prolonged hospitalizations and interventions, and causing life-threatening complications which can significantly affect the quality of life in affected individuals. It is estimated that close to 6 million Americans suffer from gastroparesis which is more common in women than men.2 The economic impact of gastroparesis can be substantial, with studies reporting 11% of patients disabled due to their gastroparesis symptoms, while another 28.5% reported a loss of yearly income.3
Nausea without slow gastric emptying may be even more common and has been referred to by many names, including chronic unexplained nausea and vomiting (CUNV), gastroparesis-like syndrome (GLS), functional vomiting, and vomiting of unexplained etiology (VUE). Many of these patients can be classified as having functional dyspepsia (FD) which is a disorder that may affect 10% of the US population.4
Non-Invasive Vagal Nerve Stimulation (nVNS) Reduces Nausea Rescue Medication in Patients with Gastroparesis and Related Disorders, with Additional Benefits on Multiple Other Associated Symptoms
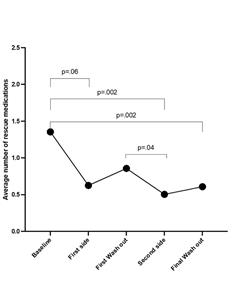
This pilot study (n=41) included patients ≥15 years of age with ongoing GP or FD symptoms for a period of ≥3 months. The primary endpoint was reducing the use of antinausea medications by using nVNS as the primary rescue treatment for nausea exacerbations. Exploratory endpoints included changes in GI, autonomic, and psychological symptoms via standardized questionnaires. The study showed nVNS reduced the use of rescue pills per day by more than 50% (p=0.0007; Figure 1). This benefit was maintained throughout each 4-week treatment period of the crossover study as well as during the two, two-week, washout periods. Exploratory endpoints revealed robust improvements in abdominal pain, reflux, indigestion, and constipation. Depression levels, as measured by the Beck Index, improved, and Migraine scores improved with the subjects using fewer headache/migraine pills per day during the study. No serious device-related adverse effects were reported.
FIGURE 1
Primary Endpoint (n=35): Average number of nausea pills. Overall significance (Type 3 F test): p=0.0007
Presentation Details:
Date:
Session: Plenary Session 4B - IBD / Obesity / Stomach
Presentation 68: Non-Invasive Vagal Nerve Stimulation Reduces Nausea Rescue Medication in Patients With Gastroparesis and Related Disorders, With Additional Benefits on Multiple Other Associated Symptom
Authors:
About electroCore, Inc.
electroCore, Inc. is a commercial stage bioelectronic medicine and wellness company dedicated to improving health through its non-invasive vagus nerve stimulation (“nVNS”) technology platform. Our focus is the commercialization of medical devices for the management and treatment of certain medical conditions and consumer product offerings utilizing nVNS to promote general wellbeing and human performance in
For more information, visit www.electrocore.com.
Forward-Looking Statements
This press release and other written and oral statements made by representatives of electroCore may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include, but are not limited to, statements about electroCore's business prospects and clinical and product development plans including potential commercial applications of gammaCore and nVNS to decrease the use of acute rescue medications for exacerbations of nausea due to Gastroparesis and Functional Dyspepsia; its pipeline or potential markets for its technologies; the timing, outcome and impact of regulatory, clinical and commercial developments; the issuance of
Contact:
ECOR Investor Relations
(973) 302-9253
investors@electrocore.com
1 Camilleri, M; Parkman HP; Shafi MA; Abell T; Gerson L. Clinical guideline: management of gastroparesis. 2013 Jan; 108 (1): 18-37.
2 Rey E, Choung RS, Schleck CD, Zinsmeister AR, Talley NJ, Locke GR III. Prevalence of hidden gastroparesis in the community: the gastroparesis“iceberg”. J Neurogastroenterol Motil. 2012;18:34–42.
3 Lacy BE, Crowell MD, Mathis C, Bauer D, Heinberg LJ. Gastroparesis: quality of life and health care utilization. J Clin Gastroenterol. 2018; 52: 20–24.
4 Harer, K; Pasricha PJ. Chronic Unexplained Nausea and Vomiting or Gastric Neuromuscular Dysfunction (GND) An Update on Nomenclature, Pathophysiology and Treatment and Relationship to Gastroparesis. 2016 Dec; 14 (4): 410-419.
5
6
7 NAMSA (
8 Nisola Consulting
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/8fcab29c-b71f-4485-a2f3-2427e9f8b2e3